|
MOLECULAR HARMONY GÁBOR NÁRAY-SZABÓ
The meaning of the word "harmony" is "agreement", "congruity", "combination or arrangement of parts to form consistent and orderly whole". Furthermore, "harmonize" is equivalent to be "agreeable in artistic effect" (Coulson et al., 1982). These everyday concepts are also very important in the world of molecules though they are described by other words. In the following we try to show that the various meanings are related in the scientific sense, too. Analysis of molecular harmony may lead to simple and general rules that are qualitative but help very much in understanding important aspects of non-covalent bonding in molecular associations. Present-day science has achieved enormous advancement in quantifying the description of natural phenomena, however, we need qualitative descriptions because mathematical formulation alone is not sufficient for the complete understanding of a natural phenomenon (Heisenberg, 1971). Qualitative rules may become an integral part of the scientist’s knowledge, and have an impact on the formulation of working hypotheses. What we need at the very end, is to bring exact thinking and emotions, two complementary ways leading to truth, closer to each other. In the world of molecules agreement or congruity means similarity,
while arrangement of parts to form an orderly whole corresponds to molecular
recognition, with a specific aspect, the phenomenon of induced fit,
which we can associate with tolerance. Scientists cannot disregard the
esthetic impact, variety and wealth of computer models of simple and complex
molecules, so we can speak about beauty in the world of molecules.
Let us discuss these aspects in some more detail.
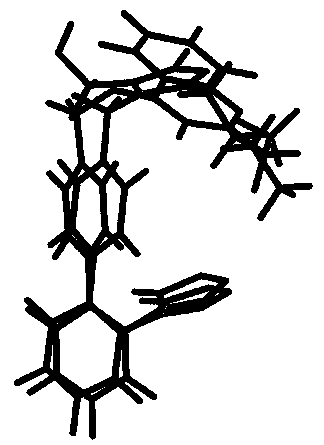
2 SIMILARITY Maybe the most important aspect of molecular similarity is related to biological activity and it is supposed that similar molecules have similar biological action. If we restrict ourselves to biopolymer-ligand binding, an event triggering a certain biological response, the meaning of similarity may be understood in terms of the lock-and-key model of Emil Fischer (1894). In this model enzymes are represented by the lock, and various keys (the bound ligands) fit to this lock. Similar, though not identical, keys may fit into the same lock leading to a similar biological response. Molecular similarity has three aspects: steric, electrostatic and hydrophobic (Náray-Szabó, 1989). On Fig. 1 we present an example for steric similarity of two molecules having about the same antihypertensive activity. It is seen that at one end, where they fit into the crevice of the target biopolymer, the molecules are quite easily superimposable, while the other ends are dissimilar.
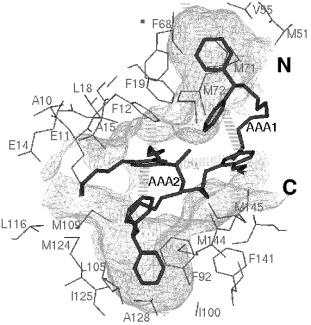
Figure 1. Steric similarity of two angiotensin II antagonist molecules . 3 MOLECULAR RECOGNITION Larger molecules with a hollow crevice specifically recognise certain smaller ones, the ligands. This means that only certain structures will fit into this crevice to trigger the biological response, others not. Molecular recognition originates in lock-and-key complementarity, which, like similarity, has three main aspects: shape, electrostatic and hydrophobic (Náray-Szabó, 1989). Steric and hydrophobic complementarity is illustrated on Fig. 2, where the hydrophobic, i.e. water repellent, arylalkylamine ligand fits nicely into the macromolecular cavity, which is surrounded by hydrophobic amino-acid residues. This is a representation of the similis simili gaudet principle (Náray-Szabó, 1989): host-guest (biopolymer-ligand) binding prefers hydrophilic/hydrophilic and hydrophobic/hydrophobic associations.
Figure 2. Complementarity between calmodulin and its arylalkylamine ligand (Harmat et al., 2000).
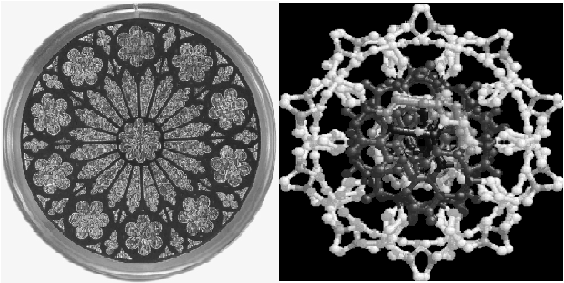
4 BEAUTY Molecules are parts of Nature, therefore, like other macroscopic objects as landscapes, plants, animals or humans, they may have a certain intrinsic beauty. An aspect of beauty is symmetry, which is nicely illustrated in a cathedral window, as well as in a cross section of the molecular model of deoxyribonucleic acid (cf. Fig.3). Tenfold symmetry is
Figure 3. Tenfold
symmetry in the Washington National Cathedral „creation" window
quite rare among cathedral windows, 6-, 8-, 12- and 16-fold symmetries
are most common. The coincidence between a window paying tribute to creation
and the molecular basis of life, deoxyribonucleic acid, is striking.
References Harmat, V., Böcskei, Z., Náray-Szabó, G., Bata, I., Csutor, A. S., Hermecz, I., Arányi, P., Szabó, B., Liliom, K., Vértessy, B. G., and Ovádi, J. (2000) A new potent calmodulin antagonist with arylalkylamine structure: Crystallographic and functional Studies, Journal of Molecular Biology 297, 747-755. Heisenberg, W. (1971) Physics and Beyond, New York: Harper and Row. Náray-Szabó, G. (1989) Electrostatic complementarity in molecular associations, Journal of Molecular Graphics, 7, 76-80. Náray-Szabó, G. (2004) Molecular harmony, European Science Open Forum, http://www.esof2004.org/ programme_events/session_papers.asp.
|